实验室目前研究方向:
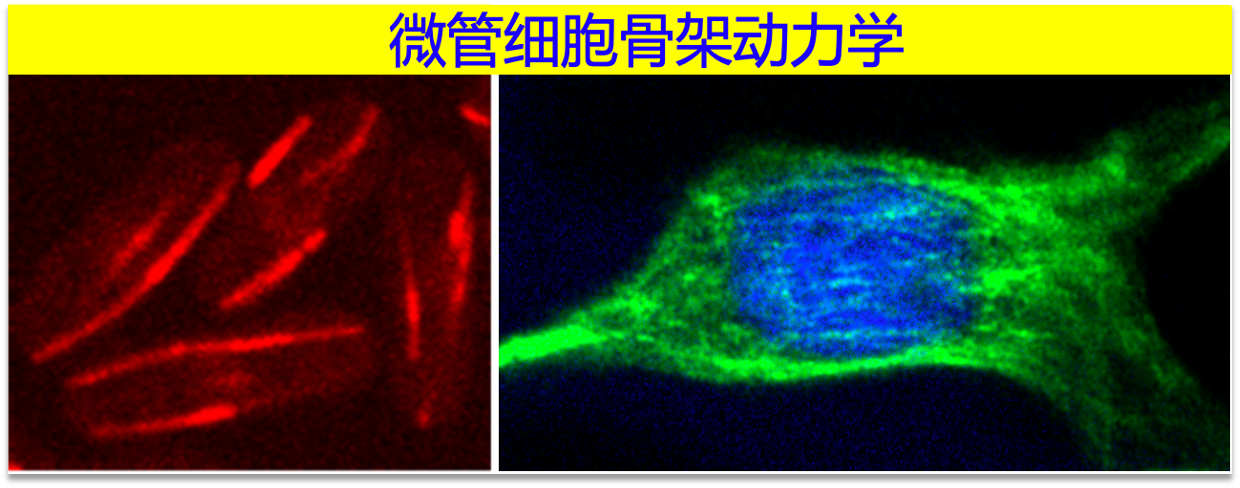
1.微管细胞骨架动力学(Microtubule dynamics)
微管是由α-,β-tubulin异二聚体经头尾相接形成的13根protofilaments通过并排连接构成的中空管状结构,直径~25nm。该结构赋予微管极性及动态的特征,使之在不同类型细胞中能形成不同微管阵列,介导广泛的细胞生物学功能。例如,参与细胞器运输、细胞极性建立、细胞迁移、有丝分裂染色体的分配和分离。许多参与调控微管细胞骨架阵列形成和动力学的微管结合蛋白(MAPs, Microtubule Associated Proteins)在肿瘤细胞中表达异常或发生突变。为此,我们的研究瞄准进化上保守的微管结合蛋白,以裂殖酵母(S.pombe)为模式生物,通过利用高时空分辨率活细胞显微镜观察分析,结合酵母遗传学的优势,重点解析微管结合蛋白调控微管动力学机制。Microtubules are hollow tubular structures composed of 13 protofilaments within which α-,β-tubulin heterodimers bind to one another in a head-to-tail fashion. This structural arrangement confers the physical properties of polarity and dynamics to microtubules and thus allows microtubules to have a wide range of cellular functions including transporting organelles, directing cell motility, and mediating chromosome segregation. It is often found that microtubule associated proteins (MAPs) are expressed abnormally, and/or mutated, in tumor cells, making MAPs highly relevant to tumorigenesis. Hence, our research efforts are focused on investigating how MAPs are involved in regulating microtubule dynamics and mediating formation of the cell-type specific microtubule arrays.
2.线粒体动力学 (Mitochondrial dynamics)
线粒体是细胞的能量工厂,生产ATP,为极为重要的细胞器。其在细胞内高度动态,面对不同的环境,通过调控自身分裂(fission)、融合(fusion)及与细胞骨架相互作用,进而呈现不同的胞内定位特征。线粒体缺陷通常与神经退行性疾病及癌症等许多重大疾病的发生和发展息息相关。因此,我们通过综合利用酵母遗传学、生物化学、超高分辨率显微镜观察成像等先进技术手段重点研究线粒体动力学调控的分子机制。
Mitochondria are the powerhouse of a cell, constantly undergoing fusion and fission and intimately interacting with the cytoskeleton. Mitochondria malfunctions are associated with neuron degenerative diseases, aging, and tumorigenesis. Employing high spatiotemporal resolution microscopy, yeast genetics, and biochemistry, we aim to reveal the molecular mechanisms underlying mitochondria dynamics and regulating microtubule-mitochondria interactions.

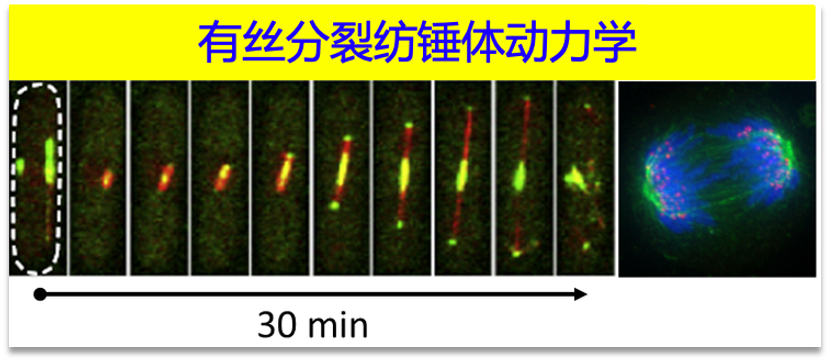
3.有丝分裂和减数分裂(Mitosis and meiosis)
姐妹染色单体通过有丝分裂均等准确地向子代细胞分配,进而维系染色体遗传的稳定性。由于有丝分裂异常可导致非整倍体细胞形成,因此有丝分裂与肿瘤的发生和发展关系紧密。我们重点剖析有丝分裂过程重要细胞器纺锤体及动点的动态组装机制和调控机理。
Mitosis is a fundamental process of life. During mitosis, chromosomes are segregated and equally divided into two daughter cells. Mitosis errors are usually correlated with failures of chromosome segregation, leading to more severe problems such as genomic instability, birth defects and cancer. My laboratory is interested in understanding the fundamental molecular mechanisms underlying spindle and chromosome dynamics during mitosis. We combine yeast genetics, quantitative live-cell imaging, biochemical and cell biological approaches to dissect organization, dynamics and regulation of the spindle in the fission yeast Schizosaccharomyces pombe.